Non-invasive MRI tools for liver fat evaluation are essential for reducing biopsies and enabling early, repeatable assessment of hepatic steatosis. Yet, despite their clinical value, these tools are rarely integrated into radiologists’ workflows and are often not optimized for real-world imaging conditions. As a result, their potential remains largely untapped — not due to lack of capability, but because they are not designed around how liver imaging is performed.
Embedded Workflow Integration
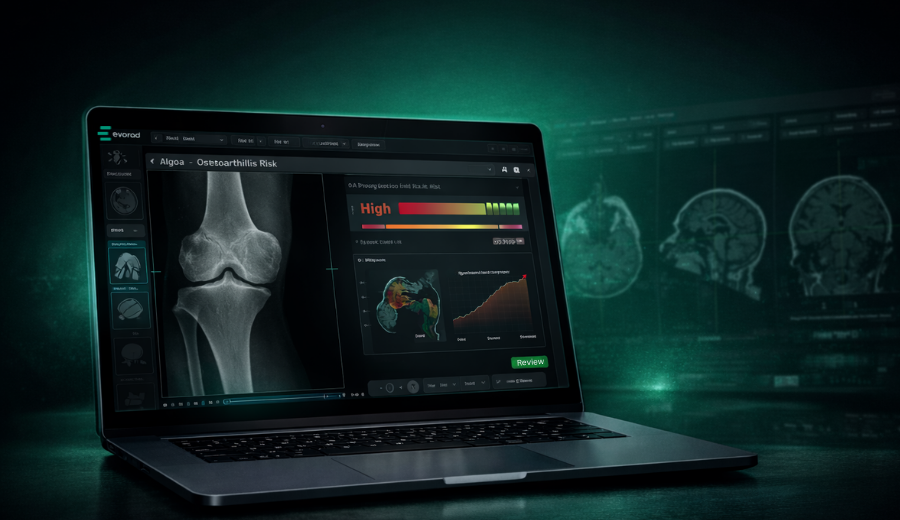
Rather than creating a detached, exclusive solution, we designed μetaRadiology to be embedded directly into PACS, where radiologists already work. By embedding liver fat evaluation into the reading flow, it becomes part of routine interpretation instead of an extra step, reducing friction and supporting consistent clinical use.
Validation is equally critical. The algorithm has been optimized and tested on in-house phantoms, ensuring consistency and robustness before deployment — turning potential into reliable, real-world performance. This added value of phantom validation is also why μetaRadiology is used in research-driven centers such as ISGlobal The emphasis is not only on flexible functionality but also on delivering liver fat evaluation that is reproducible, technically accurate, and aligned with clinical reality, enabling meaningful analysis without unnecessary complexity.
The Clinical Context: NAFLD and the Limitations of Biopsy
Beyond workflow and validation, liver fat evaluation must be viewed in the broader context of liver disease management. Non-alcoholic fatty liver disease (NAFLD) is highly prevalent in both adults and children and represents a growing global health burden. While liver biopsy has historically beenconsidered the reference standard for evaluating hepatic steatosis, it is accompanied by significant limitations. It is invasive, expensive, and subject to sampling variability, making it unsuitable for routine monitoring and longitudinal assessment [1–3].
MRI-PDFF: A Reliable Non-Invasive Alternative
MRI-derived proton density fat fraction (PDFF) has therefore emerged as a reliable non-invasive alternative for the detection and quantification of liver fat. Multiple studies have demonstrated strong correlation between MRI-PDFF and histological grading of steatosis, supporting its role in both clinical and research settings [4–5]. Importantly, non-invasive assessment enables repeatable measurements over time, which is critical for monitoring disease progression and evaluating treatment response in NAFLD [6–10, 11–13].

Early Evaluation and Clinical Decision-Making
Early and reliable evaluation of liver fat plays a key role in clinical decision-making. Monitoring changes in steatosis can inform treatment strategies, support preventive care, and help avoid adverse outcomes associated with disease progression [6–10, 14–16]. This is particularly relevant in populations where repeated invasive procedures are neither practical nor ethically desirable.

The Challenge of Consistency and Trust
However, the clinical value of MRI-based liver fat evaluation depends heavily on consistency and trust. Variability in acquisition techniques, reconstruction methods, and post-processing approaches can affect measurement reliability, especially when tools are not optimized or standardized [17–22]. From a product perspective, this variability undermines confidence and limits adoption — regardless of the underlying scientific validity [23–26].
This is why optimization and validation must be foundational rather than optional. Phantom-based optimization allows algorithms to be calibrated against known reference values under controlled conditions before being exposed to real- world variability. In practice, this approach supports reproducibility and consistency, which are essential for longitudinal assessment and clinical confidence.
Clinical Application: Color-Coded Fat Fraction Maps
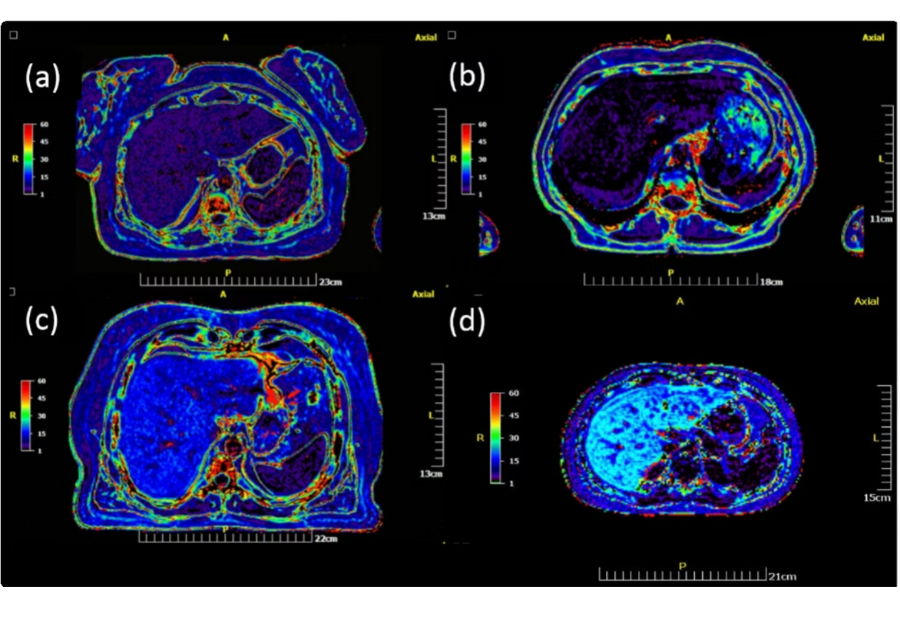
Figure 1: Color-coded liver fat fraction (%FF) maps generated using μetaRadiology for three adult patients (a–c) and one pediatric patient (d), with patient sex as follows: (a) female, (b) male, (c) male, and (d) female. Pseudo-coloring is based on the NIH color palette, with dark colors representing %FF <5 (Grade 0, Normal), and spectral colors from violet to vivid red representing %FF 5.1–50. Mean quantitative %FF values were 6.2%, 3.3%, 17.2%, and 22.4% for patients (a), (b), (c), and (d), corresponding respectively to Grade 1 (Mild), Grade 0 (Normal), Grade 2 (Moderate), and Grade 3 (Heavy) steatosis.
Embedded Delivery: Removing Barriers to Adoption
 Equally important is how evaluation is delivered. When liver fat assessment requires external software or fragmented workflows, its use becomes limited. Embedding evaluation directly into PACS removes these barriers, allowing non- invasive assessment to become part of routine imaging rather than a specialized add-on. In this setting, consistency improves naturally, because evaluation is aligned with how radiologists already work.
Equally important is how evaluation is delivered. When liver fat assessment requires external software or fragmented workflows, its use becomes limited. Embedding evaluation directly into PACS removes these barriers, allowing non- invasive assessment to become part of routine imaging rather than a specialized add-on. In this setting, consistency improves naturally, because evaluation is aligned with how radiologists already work.
Looking ahead, the future of imaging analytics is unlikely to be dominated by monolithic platforms. Instead, it will be shaped by focused, optimized tools that integrate seamlessly into existing systems and support better decisions without adding friction. Liver fat evaluation is a clear example of why this approach matters.
Beyond Measurement: Supporting Better Patient Care
When non-invasive assessment is embedded, validated, and designed around real clinical workflows, it moves beyond measurement alone. It becomes a meaningful signal, one that supports monitoring, decision-making, and ultimately better patient care.
- Embedded Integration: Seamlessly integrated into existing PACS workflows
- Validated Performance: Optimized and tested with phantom-based validation
- Clinical Workflow: Designed around how radiologists actually work
- Better Care: Supports monitoring, decisions, and patient outcomes
μetaRadiology: Beyond Images to Meaningful Information
μetaRadiology reflects the essence of its name. “μeta” — meaning “beyond” — captures the philosophy of going beyond images to deliver meaningful information. Like metadata complements data, μetaRadiology complements radiology by mapping insights, improving conspicuity, enhancing understanding of disease, and supporting clinical decisions. It turns measurement into understanding, bringing imaging closer to patient care.
References
- Janiec DJ, Jacobson ER, Freeth A, Spaulding L, Blaszyk H (2005) Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver Obes Surg 15:497–501.
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver Gastroenterology 128:1898–1906.
- Bondini S, Kleiner DE, Goodman ZD, Gramlich T, Younossi ZM (2007) Pathologic assessment of non-alcoholic fatty liver Clin Liver Dis 11:17–23, vii.
- Dulai PS, Sirlin CB, Loomba R (2016) MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical J Hepatol. doi: 10.1016/j.jhep.2016.06.005
- Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, Xin Y (2019) Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur doi: 10.1007/s00330-019-06072-4
- Wieckowska A, McCullough AJ, Feldstein AE (2007) Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and Hepatology 46:582–589.
- Kim SH, Lee JM, Han JK, Lee JY, Lee KH, Han CJ, Jo JY, Yi N-J, Suh K-S, Shin K-S, Jo SY, Choi BI (2006) Hepatic macrosteatosis: predicting appropriateness of liver donation by using MR imaging–correlation with histopathologic Radiology 240:116–129.
- McCormack L, Clavien P-A (2005) Understanding the meaning of fat in the Liver Transpl 11:137–139.
- Samara A, Ventura EE, Alfadda AA, Goran MI (2012) Use of MRI and CT for fat imaging in children and youth: What have we learned about obesity, fat distribution and metabolic disease risk? Obesity Reviews 13:723–732.
- Cho JY, Suh K-S, Kwon CH, Yi N-J, Cho SY, Jang J-J, Kim SH, Lee KU (2005) The hepatic regeneration power of mild steatotic grafts is not impaired in living-donor liver Liver Transpl 11:210–217.
- Tapper EB, Loomba R (2018) Noninvasive imaging biomarker assessment of liver fibrosis by elastography in Nat Rev Gastroenterol Hepatol 15:274– 282.
- Hu HH, Börnert P, Hernando D, Kellman P, Ma J, Reeder S, Sirlin C (2012) ISMRM workshop on fat-water separation: Insights, applications and progress in Magn Reson Med 68:378–388.
- Reeder SB, Cruite I, Hamilton G, Sirlin CB (2011) Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and J Magn Reson Imaging 34:729–749.
- Bray TJP, Chouhan MD, Punwani S, Bridge A, Hall-Craggs MA (2018) Fat fraction mapping using magnetic resonance imaging: Insight into British Journal of Radiology. doi: 10.1259/bjr.20170344
- Hines CDG, Yu H, Shimakawa A, McKenzie CA, Warner TF, Brittain JH, Reeder SB (2010) Quantification of hepatic steatosis with 3-T MR imaging: Validation in ob/ob Radiology 254:119–128.
- Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Patton HM, Sirlin CB (2009) Diagnostic and Fat-Grading Accuracy of low-flip-angle multiecho gradient recalled echo MR Imaging at 5T. Radiology 251:67–76.
- Meisamy S, Hines CDG, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB (2011) Quantification of hepatic steatosis with T1-independent, T2*-corrected MR imaging with spectral modeling of fat: Blinded comparison with MR Radiology 258:767–775.
- Boris Guiu M, Jean-Michel Petit M, Romaric Loffroy M, Douraied Ben Salem M, Serge Aho M, David Masson P, Patrick Hillon, MD P, Denis Krause, MD P, Jean-Pierre Cercueil M (2009) Quantification of Liver Fat Content : Comparison of Triple-Echo and in Vivo Proton MR Spectroscopy 1 Purpose : Methods : Results : Conclusion : 250:95–102.
- Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT (2008) Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point Dixon and three-point Magn Reson Med 59:521–527.
- Fischer MA, Raptis DA, Montani M, Graf R, Clavien PA, Nanz D, Alkadhi H, Scheffel H (2012) Liver Fat Quantification by Dual-echo MR Imaging Outperforms Traditional Histopathological Acad Radiol 19:1208–1214.
- Qayyum A, Nystrom M, Noworolski SM, Chu P, Mohanty A, Merriman R (2012) MRI steatosis grading: Development and initial validation of a color mapping American Journal of Roentgenology 198:582–588.
- Ishizaka K, Oyama N, Mito S, Sugimori H, Nakanishi M, Okuaki T, Shirato H, Terae S (2011) Comparison of 1H MR spectroscopy, 3-point DIXON, and multi- echo gradient echo for measuring hepatic fat Magnetic Resonance in Medical Sciences 10:41–48.
- Brancato V, Della Pepa G, Bozzetto L, Vitale M, Annuzzi G, Basso L, Cavaliere C, Salvatore M, Rivellese AA, Monti S (2022) Evaluation of a Whole-Liver Dixon-Based MRI Approach for Quantification of Liver Fat in Patients with Type 2 Diabetes Treated with Two Isocaloric Different Diagnostics. doi: 10.3390/diagnostics12020514
- Zhong X, Nickel MD, Kannengiesser SAR, Dale BM, Kiefer B, Bashir MR (2014) Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE Magn Reson Med 72:1353–1365.
- Wang N, Cao T, Han F, Xie Y, Zhong X, Ma S, Kwan A, Fan Z, Han H, Bi X, Noureddin M, Deshpande V, Christodoulou AG, Li D (2022) Free-breathing multitasking multi-echo MRI for whole-liver water-specific T1, proton density fat fraction, and R2 Magn Reson Med 87:120–137.
- Procter AJ, Sun JY, Malcolm PN, Toms AP (2019) Measuring liver fat fraction with complex-based chemical shift MRI: The effect of simplified sampling protocols on BMC Med Imaging 19:1–9.